Circulating tumor DNA and recurrence of esophagogastric cancer
Persons with esophagogastric adenocarcinoma who undergo neoadjuvant therapy and curative-intent surgery are unfortunately still at risk for recurrence even with pathological complete (or near-complete) treatment response. If those likely to experience recurrence could be identified early they might benefit from additional treatment such as immunotherapy. This preliminary study investigates the prognostic value of circulating tumor DNA (ctDNA) as a biomarker for predicting recurrence in patients with treated early-stage esophagogastric cancer (EGC). The authors report that ctDNA positivity during the molecular residual disease window (16 weeks) is associated with a significantly shorter recurrence-free survival, even among patients who achieved a pathologic complete response. The findings suggest that ctDNA testing could refine risk stratification for adjuvant therapy decisions, although further validation in larger cohorts is needed due to limitations such as selection bias and small sample size.
Circulating Tumor DNA as a Prognostic Biomarker for Recurrence in Patients With Locoregional Esophagogastric Cancers With a Pathologic Complete Response.
Lander EM, Aushev VN, Huffman BM, Hanna D, Dutta P, Ferguson J, Sharma S, Jurdi A, Liu MC, Eng C, Klempner SJ, Gibson MK.
Abstract
Purpose: After neoadjuvant therapy (NAT) and surgery, up to one third and one half of patients with esophagogastric adenocarcinoma with a pathologic complete response (pCR; tumor regression grade 0 [TRG-0]) and near-pCR (TRG-1) will recur, respectively. Our study aims to evaluate postoperative circulating tumor DNA (ctDNA) as a predictor of recurrence in patients with pCR or near-pCR after curative-intent neoadjuvant chemotherapy or neoadjuvant chemoradiation and surgery.
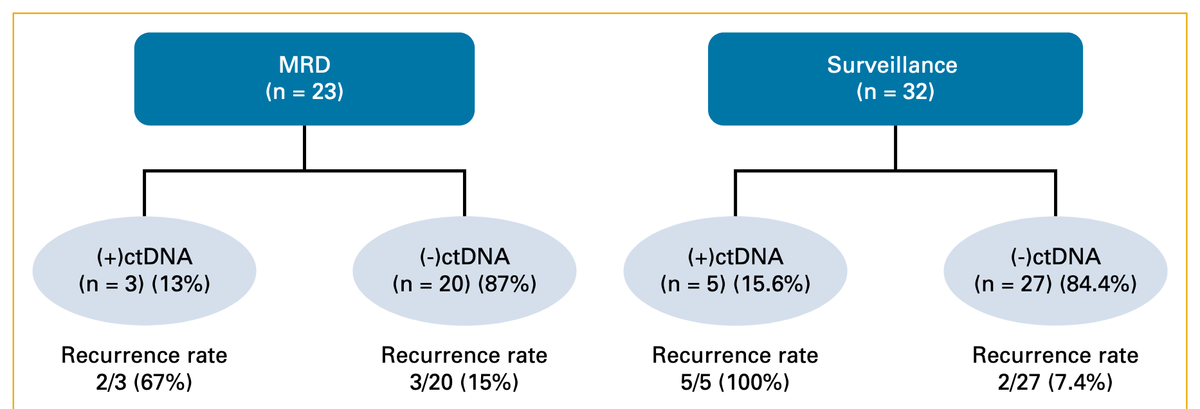
Methods: We retrospectively identified patients from 11 institutions with stages I-IV esophagogastric cancers (EGCs) who completed NAT and had TRG-0/1 scores at the time of curative-intent surgery. Postoperative plasma samples were collected for ctDNA analysis within a 16-week molecular residual disease (MRD) window after definitive surgery, and during surveillance from January 7, 2020, to November 9, 2023, at the provider's discretion. ctDNA was assessed using a clinically validated, personalized, tumor-informed ctDNA assay (Signatera, Natera, Inc). The primary outcome was recurrence-free survival (RFS).
Results: We obtained 309 blood samples from 42 patients with esophagogastric adenocarcinoma with a pCR after neoadjuvant treatment over a median follow-up time of 28.5 months (range, 0.2-81.7). Detectable ctDNA in the 16-week MRD window (N = 23) correlated with higher rates of recurrence (67%; 2/3) compared with undetectable ctDNA (15%; 3/20). Detectable ctDNA within the MRD window was associated with a significantly shorter RFS (hazard ratio [HR], 6.2; P = .049). Among 32 patients who had ctDNA analyzed in the surveillance setting, the recurrence rate was 100% (5/5) in the ctDNA-positive cohort compared with 7.4% (2/27) in ctDNA-negative patients and was associated with shorter RFS (HR, 37.6; P < .001).
Conclusion: Within the subgroup of patients with EGC and favorable pathologic responses (TRG 0-1) after NAT, the presence of postoperative ctDNA identified patients with elevated recurrence risk.