Cost-effectiveness analysis of Barrett's screening
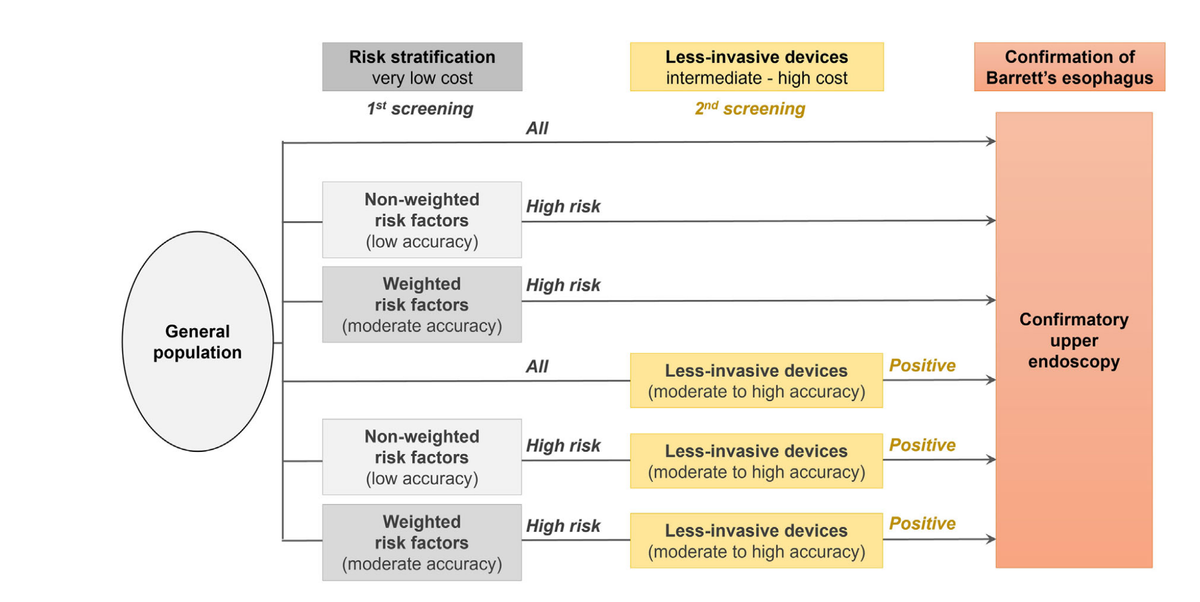
From South Australia's Flinders University, Dr. Aoki and colleagues report on a cost-effectiveness analysis of various screening strategies for identifying Barrett's esophagus (BE) in the community, The study examined four primary screening tools: non-weighted clinical risk stratification, weighted risk stratification, the Cytosponge-TFF3 device, and traditional endoscopy and analyzed two hypothetical cohorts with BE prevalence rates of 1.9% and 6.8%. The cost-effectiveness of screening strategies was measured using the incremental cost-effectiveness ratio (ICER). Device costs were found to play a critical role in determining the overall cost-effectiveness of BE screening strategies. The study revealed that at a lower prevalence of 1.9%, the costs per BE case identified are minimized when using a weighted risk stratification model followed by less-invasive devices like the Cytosponge-TFF3, and then endoscopy. This hierarchy of cost-effectiveness is contingent upon the device cost being ≤ US$600. In contrast, at a higher prevalence of 6.8% (which the authors acknowledge as probably unreasonably high), the cost threshold for devices becomes more stringent, emphasizing the need for affordable diagnostic tools to maintain cost-effectiveness.
Given the variability in community prevalence rates and device costs, the authors recommend a revision of current guidelines for Barrett's esophagus screening, advocating for the development of tailored screening strategies that consider local prevalence rates and the availability and cost of diagnostic tools. By aligning screening practices with community-specific data, healthcare providers can enhance the effectiveness and efficiency of screening programs, ultimately improving patient outcomes and resource utilization.
Cost-effective identification of Barrett's esophagus in the community: A first step towards screening.
Aoki T, Watson DI, Bulamu NB.
Abstract
The first step towards developing a screening strategy for Barrett’s esophagus (BE) is the identification of individuals in the community. Currently available tools include endoscopy, less-invasive non-endoscopic devices, and non-invasive risk stratification models. We evaluated the cost of potential strategies for identification of BE as a first step towards screening.
Methods: Two hypothetical cohorts of the general population aged ≥ 50 years with BE prevalence rates of 1.9% and 6.8% were modeled. Four potential screening tools were evaluated: (i) risk stratification based on non-weighted clinical factors according to US/European guidelines, (ii) weighted risk stratification using algorithmic models, (iii) less-invasive devices such as Cytosponge + trefoil factor 3 (TFF3), and (iv) endoscopy. Using a decision-analytic model, the cost per BE case identified and the cost-effectiveness were compared for six potential BE screening strategies based on combinations of the four screening tools; (i) + (iv), (ii) + (iv), (iii) + (iv), (i) + (iii) + (iv), (ii) + (iii) + (iv), and only (iv).
Results: The cost per BE case identified was lowest for the weighted risk stratification followed by Cytosponge-TFF3 then endoscopy strategy at both 1.9% and 6.8% BE prevalences (US$9282 and US$3406, respectively) although it was sensitive to the cost of less-invasive devices. This strategy was also most cost-effective for a BE prevalence of 1.9%. At BE prevalence of 6.8%, the Cytosponge-TFF3 followed by endoscopy strategy was most cost-effective.
Conclusions: Incorporating weighted risk stratification and less-invasive devices such as Cytosponge-TFF3 into BE screening strategies has a potential to cost-effectively identify BE in the community although device cost and the community prevalence of BE will impact the optimal strategy.